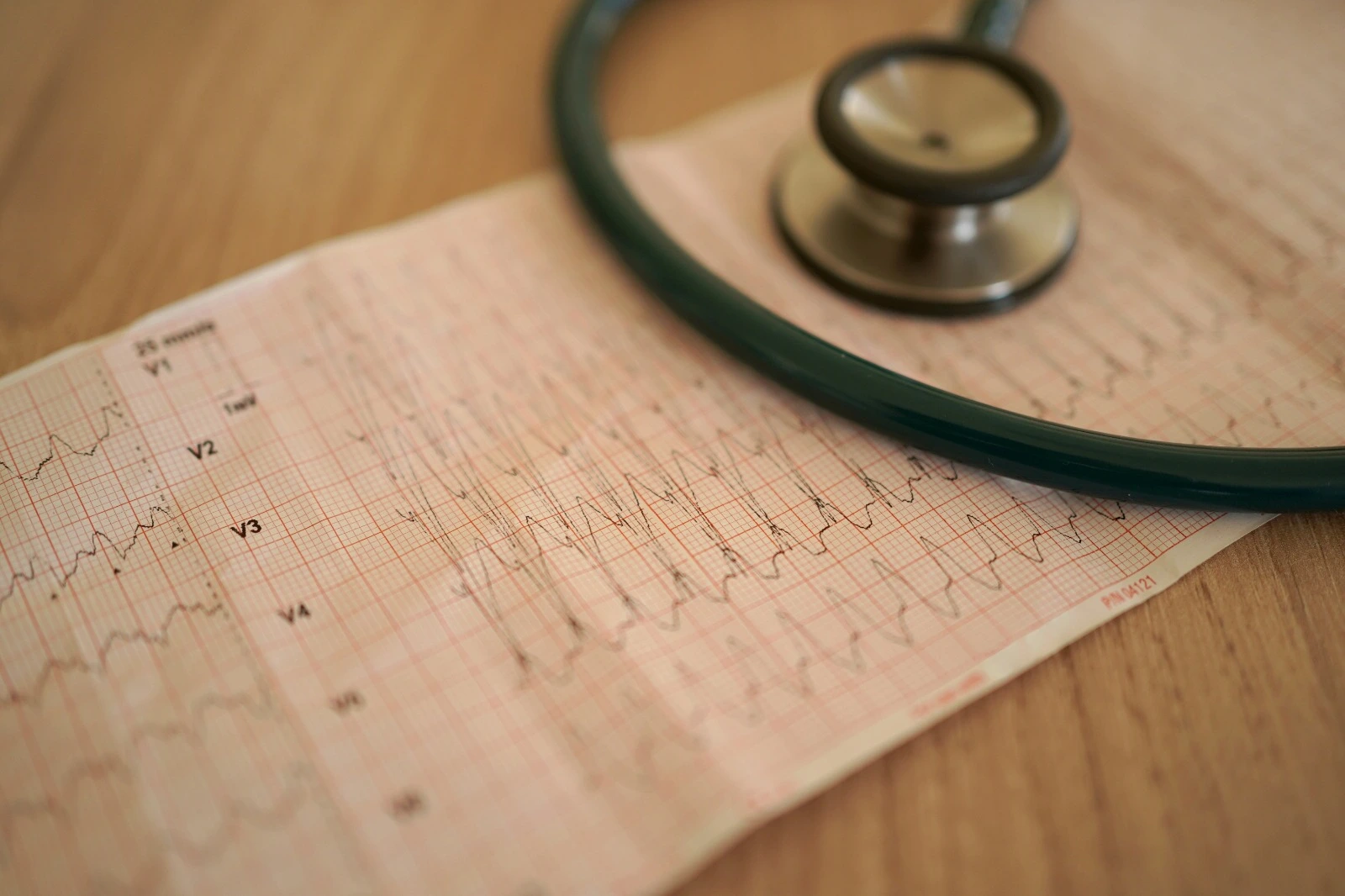
Membrane action potential consists of resting potential, depolarization, and repolarization phases, which lead to the propagation of an electrical signal to adjust cells in cardiac tissue, which occurs under the effect of electrolyte channels.
Cardiac myocyte action potential:
- Phase 0: Depolarization by Na influx through voltage-gated sodium channel (I Na) activation when depolarization exceeds threshold
- Phase 1: early rapid repolarization by transient K outflow through potassium channels (I to,f and I to,s).
- Phase 2: prolong plateau by Ca influx through a voltage-gated calcium channel (I Ca,L and I Ca,T) balanced with K outflow (I K).
- Phase 3: Repolarization when Ca channels are inactivated and K outflow becomes predominant
- Phase 4: Resting membrane potential ~ -90 mV, maintained by Na/K ATPase
Slow responsive tissues (Pacemaker cells in the Sinoatrial (SA) and Atrioventricular (AV) nodes) action potential:
- Phase 0: Depolarization by Ca inward movement (I Ca)
- Phase 3: Repolarization by slow K outer movement until reaching resting potential ~ -65 mV.
- Phase 4: Hyperpolarization by slow Na entry (I Na)
Any abnormality that affects the cardiac normal electrical system can lead to arrhythmia, including inherited factors when gene mutations affect electrolyte channels and acquired factors such as cardiomyopathy, which leads to remodeling in tissue electricity.
Featured Mechanisms:
I) Enhanced Automaticity
The sinoatrial node (SA) is the main pacemaker of the heart; it generates the highest proportion of electrical impulses, mainly under the effect of vagal tone, which usually suppresses the pace-making ability of other latent pacemaker cells (atrial myocardium and AV junction).
Enhanced automaticity usually occurs when depolarization is abnormally developed during diastole at phase 4 action potential in pacemaker cells (SA node or latent pacemaker cells), which may exceed the SA node pacemaker electrical signaling rate and lead to accelerated heart rhythm. It may be triggered by exercise, pathological hypoxia and ischemia, acidosis, digitalis toxicity, and stimulant administration.
It can occur due to abnormal action potential stimulation by non-pacemaker cells, such as myocardial cells of the atrium, Purkinje, and ventricles. As in myocardial infarction, Na/K ATPase is inactivated, thereby losing the Na channel's action potential mediation, so slow Ca channels become the action potential mediator, which leads to spontaneous automaticity. It can also be noticed in heart failure as automaticity is increased with myocardial stretching.
II) Trigger Activity
It refers to secondary depolarization that occurred after the main depolarization.
- Early after depolarizations (EADs)
If action potential is prolonged, Ca release by the sarcoplasmic reticulum or reopening of slow Ca channels can occur during phases 2 or 3, even before full action potential repolarization. It is usually associated with QT prolongation and Torsade de Point arrhythmias.
- Delay after depolarizations (DADs)
It takes place during phase 4 after full-action potential repolarization. Heart failure, hypokalemia, digoxin, and catecholamines can cause intracellular Ca overload, which activates the 3Na/1Ca exchanger and leads to transient depolarization or full action potential if the threshold is exceeded.
III) Reentry
It is the most common mechanism of tachyarrhythmias. It occurs when an action potential isn't terminated and instead reactivates a region that has recently recovered from a refractory period. This happened when electrical pathways were different in their electrophysiological properties: conduction velocity (CV) and refractory period duration (when no excitation could be caused).
1) Reentry involving an obstacle (circus type)
Under normal conditions, an electrical signal is moved through a pathway where conduction is fast but followed by a long refractory period (to prevent rapid re-excitation). This fast conduction counteracts the signal movement through any other pathway with slow conduction properties.
If a premature beat is generated, the signal cannot move through the normal fast conduction pathway, which is still in a refractory period, so it will move through the other slow conduction pathway. If it reaches the intersection point of pathways at the right time when the long refractory period is over, it will lead to signal reentry to the fast conduction pathway, then back to the slow conducting pathway, which has a short refractory period, and the circuit will be repeated over and over.
Examples of anatomical structures involved in this circuit are accessory pathways in Wolf Parkinson White Syndrome (WPWS), bundle branches, or survival branches within the infarcted myocardium.
In this case, no anatomical structure is involved in the reentry circuit. Instead, a leading circle is formed when tissues in the core are sustainedly refractory, which makes them unexcitable and leads to the reentry of electrical signals back to the same tissues. Also, a spiral wave circle may be formed when core tissues are excitable but cannot be excited and reached due to the critical curvature point during the electrical signal path. It is associated with atrial fibrillation and ventricular fibrillation.
2) Reentry not involve an obstacle
In this type, reentry occurs in linear tissue. When an electrical signal is normally conducted throughout the tissue until it reaches a very slow or depressed conduction segment, the excitability of the distant region becomes mainly dependent on the electrical current's outer-spread ability to exceed the depolarization threshold. After that, an electrical current can be formed in a retrograde direction to the proximal region through the same mechanism if it is recovered from the refractory period, and so on.
It occurs when an electrical signal is conducted from an area with a prolonged action potential duration to an area with a shorter action potential, where the later area can be re-excited if it is recovered from a refractory period. As it can be observed in Brugada syndrome.
References


.webp)
.webp)
.webp)
.webp)
.webp)

.webp)