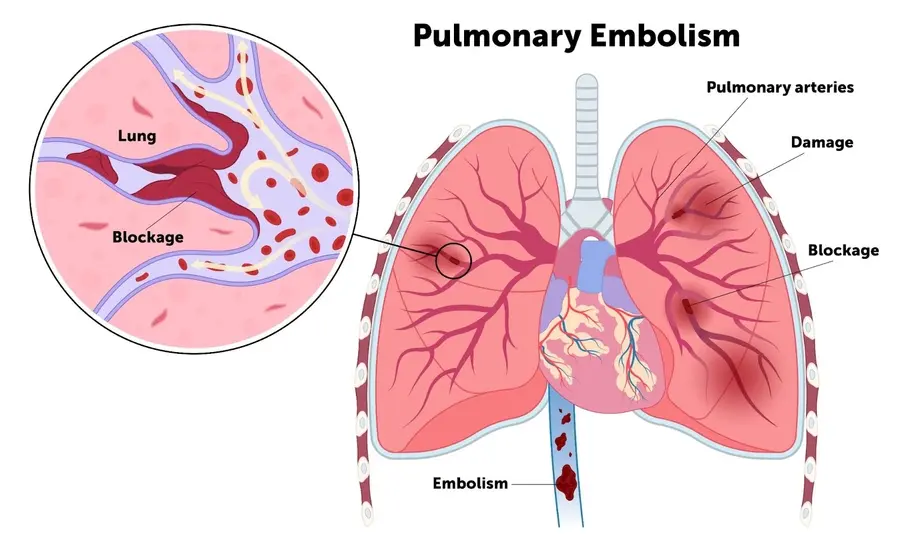
Pulmonary embolism (PE) occurs when a blood clot, known as a thrombus, obstructs an artery in the lung, disrupting blood flow to that area. Typically stemming from the deep venous system in the lower extremities, although occasionally originating in other veins such as pelvic, renal, upper extremity veins, or the right heart chambers, PE poses a risk when large thrombi migrate to the lung and lodge at critical points like the bifurcation of the main pulmonary artery or lobar branches. Such occurrences can lead to hemodynamic compromise, underscoring the gravity of this vascular condition.
Etiology
The etiology of pulmonary embolism is multifaceted and often involves a combination of factors outlined in the Virchow triad. The formation of thrombi, typically originating in the deep venous system of the lower extremities, poses a risk for embolization to the lungs. Various conditions, including venous stasis, hypercoagulable states, immobilization, surgery, trauma, pregnancy, oral contraceptives, malignancy, hereditary factors, and acute medical illnesses, contribute to the development of pulmonary embolism.
Moreover, additional risk factors such as drug abuse, hemolytic anemias, certain medications, and inflammatory conditions further increase the susceptibility to pulmonary embolism. Emphasizing the prevalence of specific risk factors in patients with pulmonary embolism underscores the importance of recognizing factors such as immobilization, recent travel, surgery, malignancy, and other predisposing conditions.
In pediatric cases, identifiable risk factors or underlying disorders are prevalent, with indwelling central venous catheters and inherited coagulation disorders being significant contributors. Long-term hyperalimentation treatment in children may also lead to a higher incidence of pulmonary embolism, particularly in the context of major thrombosis. Dehydration, especially hyperosmolar dehydration, is an observed factor in younger infants with pulmonary emboli.
Understanding the diverse etiological factors involved in pulmonary embolism is crucial for early recognition, risk assessment, and appropriate management to mitigate the potential complications associated with this serious medical condition.
Pathophysiology
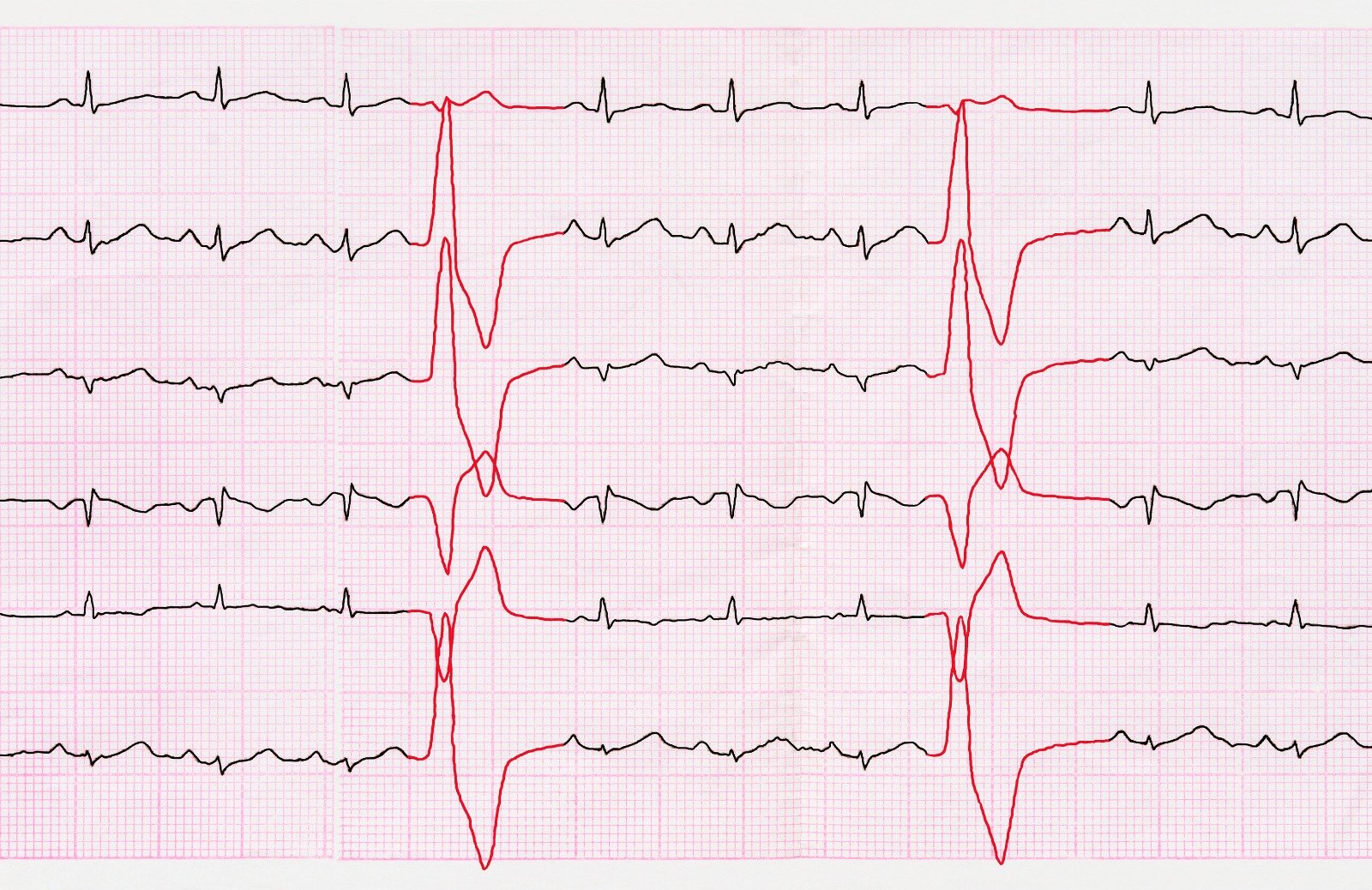
Pulmonary embolism gives rise to both respiratory and hemodynamic consequences. Acute respiratory effects involve increased alveolar dead space, hypoxemia, and hyperventilation, with additional complications such as regional loss of surfactant and pulmonary infarction. Arterial hypoxemia, though frequent, is not universal and can be attributed to various mechanisms, including ventilation-perfusion mismatch and intrapulmonary shunts.
On the hemodynamic front, pulmonary embolism diminishes the cross-sectional area of the pulmonary vascular bed, leading to heightened pulmonary vascular resistance and increased right ventricular afterload. Severe afterload elevation may result in right ventricular failure, while humoral and reflex mechanisms contribute to pulmonary arterial constriction. Anticoagulant therapy typically facilitates the rapid resolution of emboli within the first two weeks; however, residual findings may persist on chest imaging for an extended period.
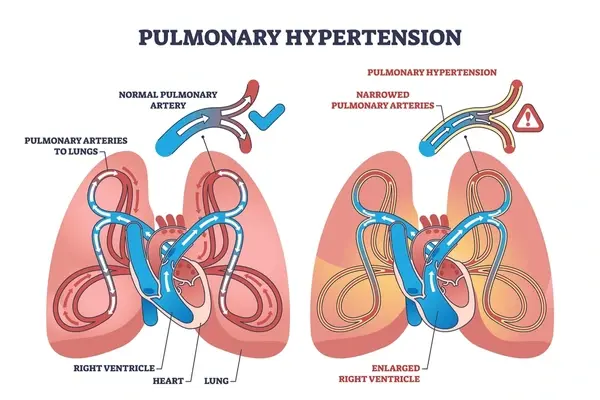
It's important to note that chronic pulmonary hypertension may develop if the initial embolus fails to undergo lysis or in the context of recurrent thromboemboli. Thus, understanding the intricate interplay of respiratory and hemodynamic consequences is crucial for effective management and prevention of complications associated with pulmonary embolism.
Clinical Presentation
The diagnosis and management of pulmonary embolism present a multifaceted challenge due to the diverse and often nonspecific clinical presentations. The history of patients with pulmonary embolism reveals a range of risk factors, from venous stasis to drug-induced factors, emphasizing the importance of a comprehensive assessment in individuals displaying symptoms or associated conditions.
The variability in physical examination findings further complicates the identification of pulmonary embolism, with different categories such as massive pulmonary embolism, acute pulmonary infarction, acute embolism without infarction, and multiple pulmonary emboli or thrombi demonstrating a spectrum of clinical manifestations. The presence of atypical symptoms, such as seizures, syncope, abdominal pain, and fever, productive cough, wheezing, decreasing level of consciousness, new onset of atrial fibrillation, underscores the need for a high index of suspicion when risk factors are present.
Furthermore, the diverse presentation in children, though less marked than in adults, requires special attention. The rarity of pulmonary embolism in pediatric cases may contribute to underdiagnosis, necessitating a nuanced approach to recognize subtle signs such as cough, tachypnea, and hemoptysis.
In the face of these challenges, a proactive approach to seek a diagnosis actively in patients with respiratory symptoms, especially in the presence of risk factors, is crucial. The incidence of physical signs, including tachypnea, rales, and fever, highlights the need for a comprehensive evaluation.
Diagnosis
- Clinical presentation of acute PE is non-specific, making a thorough assessment crucial for accurate diagnosis.
- Various clinical prediction rules, such as the revised Geneva rule and the Wells rule, aid in determining pre-test probability.
- PERC criteria help avoid unnecessary diagnostic tests in low-risk patients, contributing to cost-effectiveness.
- D-dimer testing, while having high negative predictive value, requires careful consideration of age-adjusted cut-offs and clinical probability.
- CTPA remains the preferred imaging method for suspected PE, offering high sensitivity and specificity.
- Lung scintigraphy (V/Q scan) is an alternative, especially in specific patient populations, but its limitations should be acknowledged.
- Pulmonary angiography, once the gold standard, is now rarely used due to its invasiveness and the availability of less risky alternatives.
- MRA, though promising, lacks sensitivity and availability for routine clinical use in suspected PE.
- Echocardiography plays a role in assessing RV pressure overload and dysfunction but is not mandatory in stable patients.
- CUS is essential for identifying DVT, especially in patients with suspected PE.
- CT venography during CTPA is an option, but its value and increased radiation exposure need further validation.
Recommendations for diagnosis pic
https://academic.oup.com/eurheartj/article/41/4/543/5556136#211358683
Treatment & Management
Treatment in the acute phase
- Haemodynamic and Respiratory Support:
- Oxygen therapy is crucial for hypoxaemic patients with SaO2 <90%.
- Consider high-flow oxygen or mechanical ventilation for severe hypoxaemia, especially in cases of cardiac arrest.
- Caution is needed with positive-pressure ventilation to avoid worsening RV failure.
- Pharmacological Treatment of RV Failure:
- Fluid challenge cautiously considered based on central venous pressure.
- Vasopressors like norepinephrine may be used in cardiogenic shock.
- Use of dobutamine may be considered in selected cases.
- Vasodilators should be used cautiously due to potential systemic hypoperfusion.
- Mechanical circulatory support, like ECMO, may be used in high-risk PE, but its benefits and risks should be carefully weighed.
- LMWH or fondaparinux preferred over UFH for high or intermediate clinical probability of PE.
- NOACs are effective alternatives with fixed doses and fewer interactions compared to VKAs.
- VKAs remain an option, especially in patients with pharmacogenetic considerations or those requiring self-monitoring.
- Thrombolysis is effective, especially within 48 hours of symptom onset, with careful consideration of bleeding risk.
- Catheter-directed treatment and surgical embolectomy are options for selected cases, with ECMO support showing promise.
- Multidisciplinary PE teams (PERTs) enhance rapid decision-making in severe cases.
- Use is considered in specific cases, such as absolute contraindication to anticoagulation or recurrent PE despite anticoagulation.
- Complications and risks associated with vena cava filters should be carefully considered, and their broad use requires further evidence.
Chronic treatment and prevention of recurrence
Chronic treatment and prevention of recurrent venous thromboembolism (VTE) after an acute pulmonary embolism (PE) involves a careful balance between the need for anticoagulation and the associated risk of bleeding. The following key points summarize the recommendations for long-term management:
- Duration of Anticoagulation: All patients with PE should receive a minimum of ≥3 months of anticoagulant treatment, with the decision for extended treatment based on an individual risk-benefit assessment.
- Risk Assessment for Recurrence: The risk of recurrent VTE after discontinuation of treatment is linked to the features of the index PE event. Specific groups, such as those with major transient or reversible risk factors, those with minor risk factors, those with unprovoked VTE, those with previous VTE episodes, and those with active cancer, require tailored assessment.
- Thrombophilia Testing: Testing for hereditary thrombophilia, especially in individuals with antithrombin, protein C, or protein S deficiency, homozygous factor V Leiden, or homozygous prothrombin G20210A mutation, may be considered for those with unexplained VTE at a young age (<50 years) and a strong family history.
- Bleeding Risk Assessment: The risk of bleeding during anticoagulant treatment should be assessed, considering factors such as advanced age, previous bleeding, active cancer, comorbidities, and medication interactions. Regular reassessment is recommended.
- Non-Vitamin K Antagonist Oral Anticoagulants (NOACs): NOACs, including dabigatran, rivaroxaban, and apixaban, have shown efficacy in preventing recurrent VTE. However, the decision to use NOACs over vitamin K antagonists (VKAs) should be based on a careful evaluation of bleeding risk, patient preference, and specific clinical circumstances.
- Extended Treatment with Aspirin: In certain cases, extended therapy with aspirin may be considered, particularly in patients at high bleeding risk, although recent evidence suggests that NOACs may be more effective than aspirin for secondary prophylaxis.
- Alternative Therapies: Trials with sulodexide demonstrated a reduction in recurrence risk without a significant increase in bleeding events, providing an alternative option for selected patients.
- Patient Involvement: In the decision-making process, active involvement of the patient is crucial to optimize treatment adherence and tailor the anticoagulant regimen to individual needs.
Recommendations for the regimen and duration of anticoagulation after pulmonary embolism in patients without cancer
https://academic.oup.com/eurheartj/article/41/4/543/5556136#211358821
References:


.webp)
.webp)
.webp)
.webp)
.webp)

.webp)